AVOID THE STEM CELL SCAM
How to Avoid Stem-Cell Therapy Scams and Understand What You need to Know About Protecting Yourself and Others
Here is another example of marketing practices in this HCTP 361 FDA Registered company add cleverly inserting, in a particular sequence, the “FDA, “361” and “Approved “ without even mentioning the word, “ Registered!” It refers to the FDA and it’s 361 designation as a HCTP, but conveniently leaves out the word, “ Registered, “ and the 361 FDA Registered HCTP that it is and instead, inserts the word, “ Approved, “ as if inferring that these products are 351 FDA Approved Drugs!
Also, if these HCTP’s are so helpful to people that they are allowed to call to themselves “stem cell therapy products,” without containing any living stem cells, per their FDA 361 Registered HCTP status, why do the companies like this one and the FDA ReCalled 361 FDA Registered HCTP company, Liveyon, employ such deeply deceptive language practices so as to confuse people? Why all the seductive, subliminal cinematography as in Liveyon’s commercials if the product actually works?
In our case, both the MD and the DC who sold And injected Liveyon into 3 of our family members, did so only because they lied to us telling us that their product contains actual living stem cells before we understood that by being only 361 FDA Registered, that it means that there are no living stem cells in the product. Did these doctors knowingly lie to us to sell their product? We experienced HOW they did it and we believe so. Why are such cunning salesmen that would abuse the privilege of their licenses
( MD / DC) by telling lies about what their product contained in order to get people to buy it?
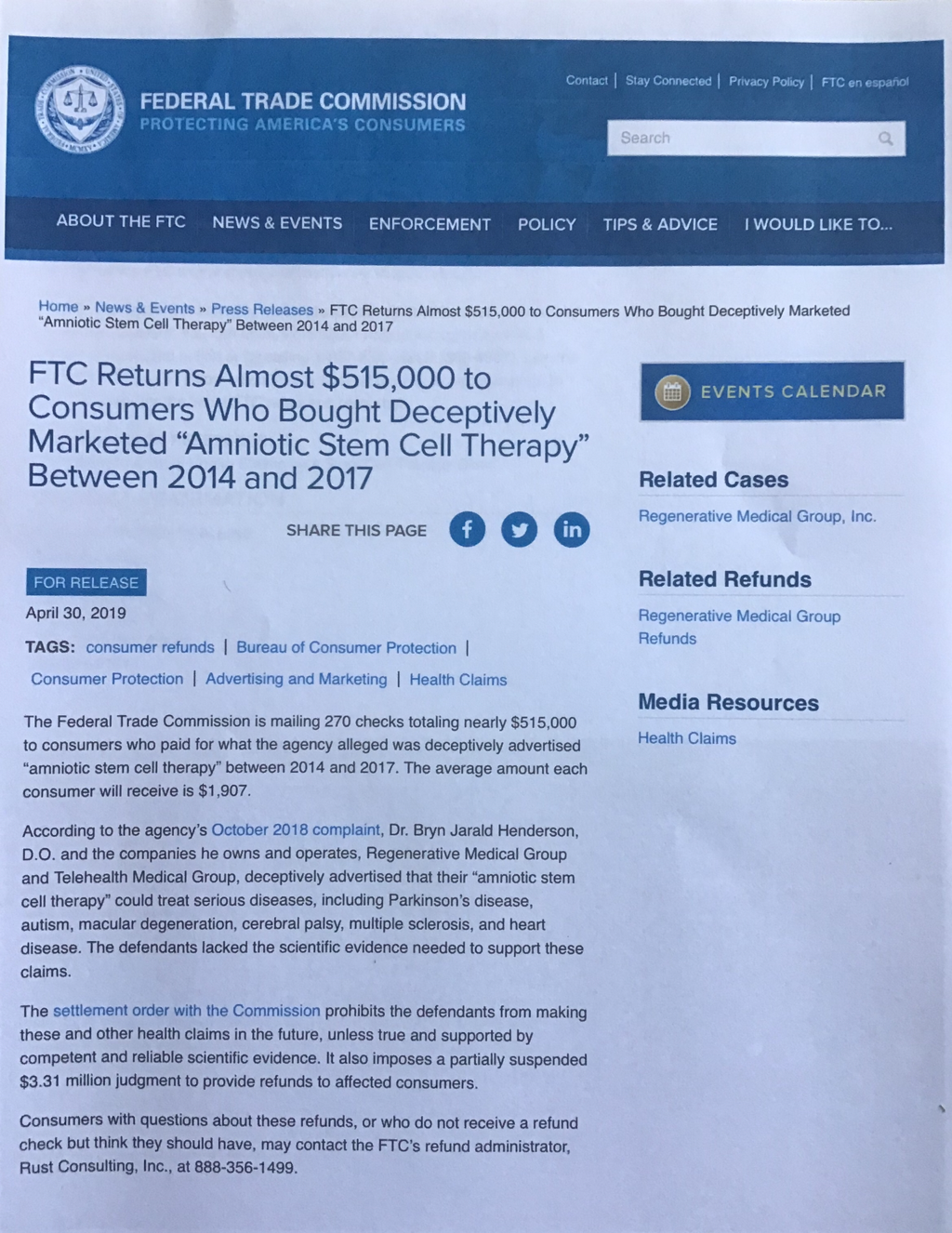
Even one digit or one letter being added or subtracted, such as in 361 vs 351, FDA Registered vs FDA Approved and even down to Liveyon’s FDA ReCalled Product that was contaminated and being sold unlawfully according to the CDC’s MMWR September 2018 report ,it’s creator, Genetech and Liveyon not Genentech.
Also, if these HCTP’s are so helpful to people that they are allowed to call to themselves “stem cell therapy products,” without containing any living stem cells, per their FDA 361 Registered HCTP status, why do the companies like this one and the FDA ReCalled 361 FDA Registered HCTP company, Liveyon, employ such deeply deceptive language practices so as to confuse people? Why all the seductive, subliminal cinematography as in Liveyon’s commercials if the product actually works?
In our case, both the MD and the DC who sold And injected Liveyon into 3 of our family members, did so only because they lied to us telling us that their product contains actual living stem cells before we understood that by being only 361 FDA Registered, that it means that there are no living stem cells in the product. Did these doctors knowingly lie to us to sell their product? We experienced HOW they did it and we believe so. Why are such cunning salesmen that would abuse the privilege of their licenses
( MD / DC) by telling lies about what their product contained in order to get people to buy it?
Even one digit or one letter being added or subtracted, such as in 361 vs 351, FDA Registered vs FDA Approved and even down to Liveyon’s FDA ReCalled Product that was contaminated and being sold unlawfully according to the CDC’s MMWR September 2018 report ,it’s creator, Genetech and Liveyon not Genentech.
Our Personal Journey Through The "Wild-West" of "Stem-Cell Therapy"... And Important Issues to Consider BEFORE Entertaining Anything referred to as "Stem-Cell Therapy"
Three simple distinctions that helped us realize that we had been scammed:
Aside from the fact that 3 of our family members had undergone a purported, "stem-cell therapy" treatment at the price of $30,000 + and NONE of us had gotten any results, except for the elderly one who had received 2 slow push IVs of a now FDA Re-Called product that landed him in the hospital with 3 surgeries including a ruptured intestinal lining, atrial fib requiring a pacemaker and a toe amputation, as well as an infected leg and foot shortly after the IVs. We began to dig for more information as to WHY the "stem-cell therapy" did not work and was possibly causing more harm. We learned that just because a product or multiple products are being sold and are being called or referred to as "stem-cell therapy" does not mean that they actually contain any living stem cells at all!
How is it that a product can possibly be called "stem-cell therapy" if it does not contain living stem cells? That is a great question! From our family experience, we think that maybe that is a great buzz word to market Human Cellular Tissue Products (HCTPs) that are only 361 FDA Registered as IF they are 351 FDA Approved Drugs that contain living stem cells because that is how my family was sold "stem-cell therapy" by the DC and MD telling us that their product contained living stem cells! We now know that these unproven and untested Human Cellular Tissue Products (HCTPs) that were sold to us as "living stem cells" when they, in fact, are NOT, nor do they contain any living stem cells (see FDA Guidelines for distinctions of what comprises a 361 FDA Registered HCTPs vs Stem Cell Therapy Drugs that are 351 FDA Approved) or they would have to be FDA 351 Approved Drugs. In addition, only FDA 351 Approved Drugs actually contain living stem cells and drugs with living stem cells are only approved in blood to blood transfers. The 361 Registered HCTP does not to "magically morph" into cartilage, annular discs, repair joint tissue or regrow damaged nerves or tissue as the chiropractor and doctor said that their now FDA RE-Called 361 Registered Human Cellular Tissue Product (HCTP) would do. This is HOW these debauched snake oil salesmen, abusing their designations as a DC and a MD, sell their product. After all, would you buy a dead birth tissue product that contained zero living stem cells that had been irradiated to go into your body expecting it to heal something?
Finding out the name of the product referred to as "stem-cell therapy" and looking up the investment side of what is being sold is wise! We found out the name of the product used on our family and called the "Investment Opportunity" site for the now FDA Recalled 361 FDA Registered HCTP called Liveyon and found a company that called itself REGENPATH (who sold Liveyon products to investors) and RegenPath explained that investors could make between $5000 and $7500 per injection site with many people getting multiple injections and/or IV's. When we asked the salesman, who said that his name was, John, if the product contained living stem cells, the answer was, "well, it is more of a perception based marketing product than a reality based product, but it does contain living stem cells and the product is viable."
On a side note, upon further investigation, the founder and CEO of Liveyon, has been involved in several court debacles. Researching the background of the founder and CEO of these purported "stem-cell therapies" as well as their constituents is advised based on our personal experiences. The Liveyon team seems to only be comprised of questionable people with judicial issues involving revocations of professional licenses and being barred from other industries and this case might suggest racketeering multiple industries for a profit by unscrupulous sales sharks.
It is also interesting to note that when the Founder/CEO of Liveyon is Googled in IMAGES, John Kosolchareon, as well as Alan Gaveck, MD, the spokes person for Liveyon on their you tube videos, and Lynne Pirie, now, another promoter of Liveyon's Re-Branded product called "PURE," as well as another guy known as Ron Chislom, who is listed as a distributor for Liveyon on Link-ed IN, but, the same photograph is listed again as John Kosolchareon. The photographs are mixed up with each others names so that one is NOT readily able to identify exactly who is whom. For example, researching John Kosolochareon, you will find pictures of 3 different men of varying demographics and nationalities, all with the same educational background. Plus, if you google Alan Gaveck, there are videos with a man marketing Liveyon and there is a photograph of a completely different looking man listed as Alan Gaveck who's photograph is also listed as being John Kosolchareon as well.
One thing that they all have in common, whomever John Kosolchareon, Alan Gaveck and Lynne Pirie really are, is that the names all have court cases involving license revocation or probation and being barred from specific industries as in the case of John Kosolochareon. The cases of each are online for your perusal.
Google search to view images of these people and draw your own conclusions.
Aside from the fact that 3 of our family members had undergone a purported, "stem-cell therapy" treatment at the price of $30,000 + and NONE of us had gotten any results, except for the elderly one who had received 2 slow push IVs of a now FDA Re-Called product that landed him in the hospital with 3 surgeries including a ruptured intestinal lining, atrial fib requiring a pacemaker and a toe amputation, as well as an infected leg and foot shortly after the IVs. We began to dig for more information as to WHY the "stem-cell therapy" did not work and was possibly causing more harm. We learned that just because a product or multiple products are being sold and are being called or referred to as "stem-cell therapy" does not mean that they actually contain any living stem cells at all!
How is it that a product can possibly be called "stem-cell therapy" if it does not contain living stem cells? That is a great question! From our family experience, we think that maybe that is a great buzz word to market Human Cellular Tissue Products (HCTPs) that are only 361 FDA Registered as IF they are 351 FDA Approved Drugs that contain living stem cells because that is how my family was sold "stem-cell therapy" by the DC and MD telling us that their product contained living stem cells! We now know that these unproven and untested Human Cellular Tissue Products (HCTPs) that were sold to us as "living stem cells" when they, in fact, are NOT, nor do they contain any living stem cells (see FDA Guidelines for distinctions of what comprises a 361 FDA Registered HCTPs vs Stem Cell Therapy Drugs that are 351 FDA Approved) or they would have to be FDA 351 Approved Drugs. In addition, only FDA 351 Approved Drugs actually contain living stem cells and drugs with living stem cells are only approved in blood to blood transfers. The 361 Registered HCTP does not to "magically morph" into cartilage, annular discs, repair joint tissue or regrow damaged nerves or tissue as the chiropractor and doctor said that their now FDA RE-Called 361 Registered Human Cellular Tissue Product (HCTP) would do. This is HOW these debauched snake oil salesmen, abusing their designations as a DC and a MD, sell their product. After all, would you buy a dead birth tissue product that contained zero living stem cells that had been irradiated to go into your body expecting it to heal something?
Finding out the name of the product referred to as "stem-cell therapy" and looking up the investment side of what is being sold is wise! We found out the name of the product used on our family and called the "Investment Opportunity" site for the now FDA Recalled 361 FDA Registered HCTP called Liveyon and found a company that called itself REGENPATH (who sold Liveyon products to investors) and RegenPath explained that investors could make between $5000 and $7500 per injection site with many people getting multiple injections and/or IV's. When we asked the salesman, who said that his name was, John, if the product contained living stem cells, the answer was, "well, it is more of a perception based marketing product than a reality based product, but it does contain living stem cells and the product is viable."
On a side note, upon further investigation, the founder and CEO of Liveyon, has been involved in several court debacles. Researching the background of the founder and CEO of these purported "stem-cell therapies" as well as their constituents is advised based on our personal experiences. The Liveyon team seems to only be comprised of questionable people with judicial issues involving revocations of professional licenses and being barred from other industries and this case might suggest racketeering multiple industries for a profit by unscrupulous sales sharks.
It is also interesting to note that when the Founder/CEO of Liveyon is Googled in IMAGES, John Kosolchareon, as well as Alan Gaveck, MD, the spokes person for Liveyon on their you tube videos, and Lynne Pirie, now, another promoter of Liveyon's Re-Branded product called "PURE," as well as another guy known as Ron Chislom, who is listed as a distributor for Liveyon on Link-ed IN, but, the same photograph is listed again as John Kosolchareon. The photographs are mixed up with each others names so that one is NOT readily able to identify exactly who is whom. For example, researching John Kosolochareon, you will find pictures of 3 different men of varying demographics and nationalities, all with the same educational background. Plus, if you google Alan Gaveck, there are videos with a man marketing Liveyon and there is a photograph of a completely different looking man listed as Alan Gaveck who's photograph is also listed as being John Kosolchareon as well.
One thing that they all have in common, whomever John Kosolchareon, Alan Gaveck and Lynne Pirie really are, is that the names all have court cases involving license revocation or probation and being barred from specific industries as in the case of John Kosolochareon. The cases of each are online for your perusal.
Google search to view images of these people and draw your own conclusions.
I've blogged quite a bit about the birth tissue products claiming to be "stem cell" rich cocktails ready for injection. While there are many companies pushing the envelope of reality in this space, one of the splashiest is Liveyon. However, recently the group went through a product recall for bacterial contamination and I thought they might come clean when they introduced their new "PURE" line of products. I was sadly disappointed. Let me explain.
Then came umbilical cord blood. The claims also were made that these were stem cell products and again, we tested this and found this to be false. In fact, it would be rare that umbilical cords would be expected to have many mesenchymal stem cells. See my video below for more info on that topic:
Liveyon Enters the Picture a few years back, Liveyon entered the picture selling umbilical cord blood and claiming that it had many live stem cells. What was interesting was that unlike some of the other companies making similar claims, Liveyon's ads had a serious artistic flair, a bit like a cross between a cosmetics line and a lifestyle brand. More importantly, I blogged on the white papers the company produced. I clearly demonstrated that while to the uneducated eye they seemed to show that there were live stem cells in the Liveyon product, the science supporting that conclusion wasn't there.
Liveyon's Contamination ProblemsA few months ago, I reported that Liveyon had issued a voluntary recall of its products after several reports of patient bacterial contamination and sepsis. At this point, I learned that Liveyon was merely a marketing company, meaning that its manufacturing was done by a company in California called Genetech. Others also reported on this contamination and the lawsuits that followed.
Would Liveyon Change It's Tune?Liveyon's founder and CEO has had legal troubles in the past and with the FDA recall, their website went dark. I believed that when they re-entered the market, that they would come clean and become one of the regulatory compliant companies that talk about the growth factor content of these tissues and who don't violate the FDA regulations by claiming that these products have live stem cells. Why does this claim violate the law? Please watch my video below to learn more:
Would Liveyon Change It's Tune?Liveyon's founder and CEO has had legal troubles in the past and with the FDA recall, their website went dark. I believed that when they re-entered the market, that they would come clean and become one of the regulatory compliant companies that talk about the growth factor content of these tissues and who don't violate the FDA regulations by claiming that these products have live stem cells. Why does this claim violate the law? Please watch my video below to learn more:
The New Liveyon "PURE" SeriesRecently I saw that Liveyon's website was back up. I have to say that I was flabbergasted. I had expected Liveyon to stop making claims about stem cells. Did that happen? Let's review what's on the site.
Well, the splashy is still there. I do have to hand it their ad agency, this is really cool stock imagery.
Well, the splashy is still there. I do have to hand it their ad agency, this is really cool stock imagery.
One of the things that's emphasized on the Liveyon site is that the cellular solution is clear, yet contains 30 million nucleated cells. In fact, the pictures show a product that appears to be as clear as water. We also read, "If the glowing clear color of the vial and perfectly transparent cellular solution inside isn’t enough to help us solidify the point, allow us to elaborate on a more scientific level, as to what went into the bio-engineering of the Pure® product. " This is puzzling to me, as I recently demonstrated what 20 million mesenchymal stem cells looked like in 1 cc of platelet lysate (an otherwise clear amber solution). Check that out below for comparison:
Meaning, as my science team agrees, you can't pack 20 or 30 million stem or nucleated cells in a 1-2 cc vial of solution and have it be clear. First, the cells will always want to settle out, which would make the bottom of any vial full of whitish gunk. If you try to suspend the cells by shaking, they will cause the liquid to be cloudy. This is the simple physics of tens of millions of cells in the 6-30 micron range. So I have no idea how Liveyon could claim that their product both has 30 million cells and is clear as water.
Next up are the continued stem cell claims. First, we read. "Scientifically validated to be the most “Pure” stem cell offering on the market to date." If you download the PDF which is the Liveyon product catalog, you are greeted by the "Liveyon LUMA LIFT, Stem Cell Enhanced Skin Treatment". So they're sticking to the idea that Liveyon has stem cells.
Even the Employees Advertise Liveyon as a Stem Cell ProductI went on Linkedin and tried to find out who was working for Liveyon. I was surprised that even the employees and contractors for this company are also boldly advertising or suggesting that Liveyon is a stem cell product. For example, Dean Brior states that he is "Regional Director for Liveyon Stem Cell Therapy" Steve Mangar states that his job title is "Stem Cell Therapy Sales" and that he is currently in sales at Liveyon. Mike Tillman states that he is with "Rejuva Stem Cell Clinics LLC" and also vice president of Liveyon LLC. The list goes on...
What is Clinical Trials Grade?Scattered throughout the website is a notion that Liveyon is a "clinical trials grade" product. In addition, the video claims that Liveyon has aspirations to get it's product FDA approved. Why are those aspirations? Because it's not FDA approved right now, but merely registered with a 45-minute free online 361 form. Meaning, there is no FDA review of the product once that form is submitted, merely the right for FDA to show up and expect the manufacturing lab to ensure that it's following tissue processing standards.
This is the conundrum of Liveyon and every other birth tissues vendor that claims live stem cells. if you claim that what you're selling is a live stem cell product, even if that claim isn't true, you must go through full FDA drug trials to prove safety and efficacy BEFORE the product can be sold. Meaning your 361 tissue registration isn't the correct regulatory category for your product. The video above explains this more thoroughly. If you don't claim that you have live stem cells, sales lag.
So what is "clinical trials grade"? This seems to be a reference to Liveyon's FDA approval aspirations. However, I can't find much information on this phrase, as it doesn't bring back anything on a simple Google search. Hence, the term seems to be made up by the company.
The upshot? Liveyon hasn't come clean with the idea that it's NOT selling stem cells. In fact, it seems to have doubled down on the concept. I'm not sure that's wise from a regulatory standpoint and I have no idea how you suspend 30 million cells in a clear liquid, so I can't wait to test a vial of the new PURE series in our advanced research lab.
Next up are the continued stem cell claims. First, we read. "Scientifically validated to be the most “Pure” stem cell offering on the market to date." If you download the PDF which is the Liveyon product catalog, you are greeted by the "Liveyon LUMA LIFT, Stem Cell Enhanced Skin Treatment". So they're sticking to the idea that Liveyon has stem cells.
Even the Employees Advertise Liveyon as a Stem Cell ProductI went on Linkedin and tried to find out who was working for Liveyon. I was surprised that even the employees and contractors for this company are also boldly advertising or suggesting that Liveyon is a stem cell product. For example, Dean Brior states that he is "Regional Director for Liveyon Stem Cell Therapy" Steve Mangar states that his job title is "Stem Cell Therapy Sales" and that he is currently in sales at Liveyon. Mike Tillman states that he is with "Rejuva Stem Cell Clinics LLC" and also vice president of Liveyon LLC. The list goes on...
What is Clinical Trials Grade?Scattered throughout the website is a notion that Liveyon is a "clinical trials grade" product. In addition, the video claims that Liveyon has aspirations to get it's product FDA approved. Why are those aspirations? Because it's not FDA approved right now, but merely registered with a 45-minute free online 361 form. Meaning, there is no FDA review of the product once that form is submitted, merely the right for FDA to show up and expect the manufacturing lab to ensure that it's following tissue processing standards.
This is the conundrum of Liveyon and every other birth tissues vendor that claims live stem cells. if you claim that what you're selling is a live stem cell product, even if that claim isn't true, you must go through full FDA drug trials to prove safety and efficacy BEFORE the product can be sold. Meaning your 361 tissue registration isn't the correct regulatory category for your product. The video above explains this more thoroughly. If you don't claim that you have live stem cells, sales lag.
So what is "clinical trials grade"? This seems to be a reference to Liveyon's FDA approval aspirations. However, I can't find much information on this phrase, as it doesn't bring back anything on a simple Google search. Hence, the term seems to be made up by the company.
The upshot? Liveyon hasn't come clean with the idea that it's NOT selling stem cells. In fact, it seems to have doubled down on the concept. I'm not sure that's wise from a regulatory standpoint and I have no idea how you suspend 30 million cells in a clear liquid, so I can't wait to test a vial of the new PURE series in our advanced research lab.